Falling film evaporator
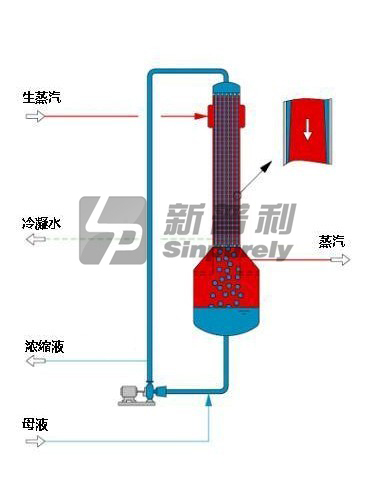
The falling film evaporator is particularly used in application, when the driving force between heat transfer medium and liquid is small.
In a falling film evaporator with separate vapor body and heat exchanger the liquor is fed into the top liquor chamber of the heat exchanger where it is distributed to each tube. The liquor accelerates in velocity as it descends inside the tubes because of the gravity and the drag of the vapor generated by boiling. Liquid is separated from the vapors in the bottom liquor chamber of the heat exchanger. The concentrated liquor is discharged from the bottom cone of the vapor body.
Evaporation occurs inside the tubes of the falling-film evaporator. The unit can be used to concentrate the same non-salting liquids concentrated in rising-film evaporators, and it is suitable for concentrating more viscous liquors.
The retention time for liquor in these evaporators is less than that for a rising-film evaporator. The combination of short liquor retention time and the ability to operate at a low dT makes the falling-film evaporator ideal for concentrating the most heat-sensitive materials.
Rising film evaporator
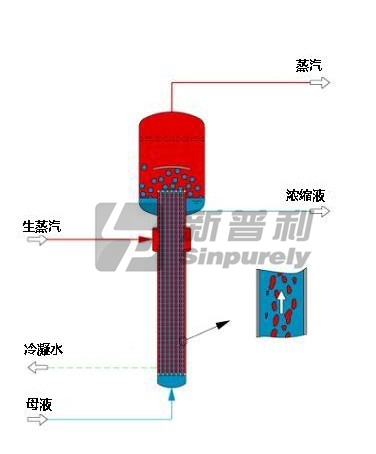
A rising film evaporator is primarily used to concentrate non-salting and non-scaling liquors.
If a high increase of concentration in one evaporator stage is required, the rising film can be the optional choice. The rising film unit is a very simple and economical device with high heat transfer rates and high availabilities. Operation of the rising-film evaporator is straightforward. Liquor is fed into bottom of the heater. There it is heated with steam or any other suitable heat medium. If the vapor pressure of the feed equals or exceeds the system pressure, vaporization will occur immediately.
The liquor climbs up inside of the tubes and therefore additional vapor is generated and the velocity of the liquor-vapor mixture increases to a maximum at the end of the tubes. The liquor-vapor separation takes place in the evaporation body by gravity and by an entrainment separator.
Forced-circulation evaporator
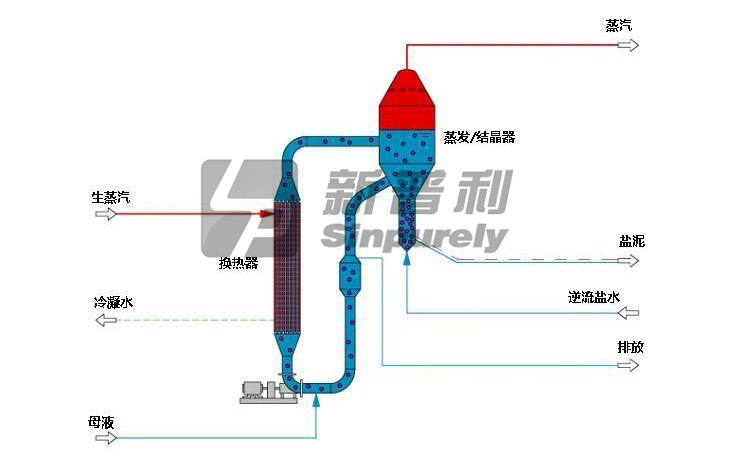
As the name implies, the liquor in a forced-circulation evaporator is pumped through tubes to minimize tubes scaling or salting when precipitates are formed during evaporation.
Slurry is pumped from the bottom cone of the vapor body through the tubes of the heat exchanger, where heat is added, and back into the vapor body where evaporation occurs. Sufficient liquor height is maintained above the heat exchanger to suppress boiling in the inlet and prevent surface boiling on the tube surface. A high circulation rate is provided for adequate tube velocity to achieve good heat transfer. A sufficient quantity of salt crystals is suspended in the circulating system to provide seed crystals in the boiling zone for salt growth. Adherence to these basic principles of crystallization results in coarse crystals and minimal wall and tube scaling.
OSLO evaporator
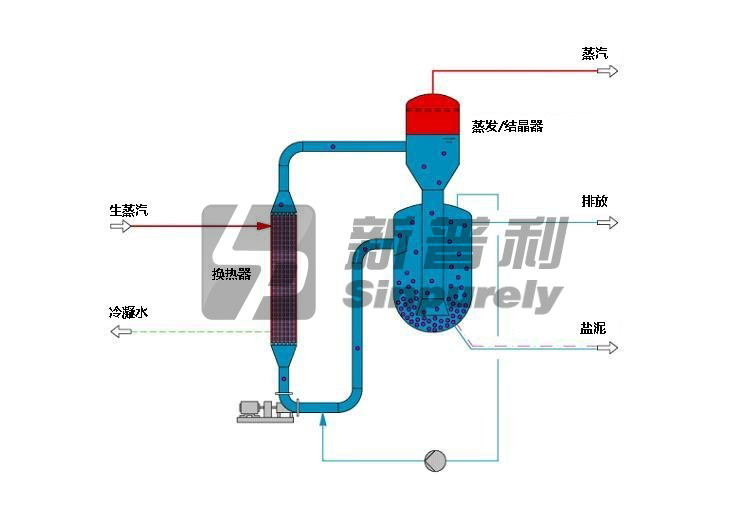
Where crystal growth is critical, our CSMPR (Classified Suspension Mixed Product Removal) growth type crystallizer can be applied.
The growth type crystallizer consists of a concentric two-body arrangement connected by a central down comer. The lower body (retention or growth chamber) supplies the necessary volume and fluidization characteristics for developed super saturation release and crystal growth. The super saturation is released upon a fluidized bed of crystals classified by particle size resulting in controlled crystal growth. By removing the crystalline products from a specific location in the classified bed, a distinct crystal size can be targeted.
The upper body (vapor body) is used for evaporation applications where sufficient surface area and disengagement height is required for vapor release. An external pump provides recirculation from the retention chamber through the heat exchanger.
Our growth type crystallizer can produce a narrow distribution of larger crystals. Typical application for these systems can include ammonium sulfate, ammonium nitrate and potassium chloride.